PHOTO
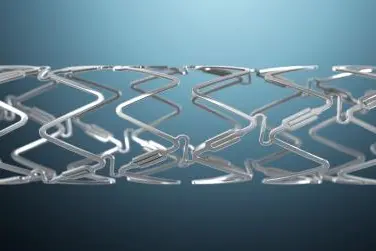
Kuwait – Elixir Medical, a developer of advanced systems to treat coronary artery disease, announced the launch of the DynamX™ Coronary Bioadaptor System at centers of excellence in the Middle East. DynamX is an innovative device that enables physicians to treat a blockage of the coronary artery while freeing the artery to return to its normal function by adapting to vessel physiology.
Cardiovascular diseases (CDA) are on the rise in the region and are responsible for 34% of all deaths in the Middle East population.[1] In Kuwait, coronary artery disease accounted for 33% of all deaths, according to data from the World Health Organization published in 2018.[2] As Kuwait is in the forefront of adopting smart technology to better the life of its citizens, the use of the DynamX Bioadaptor will complement the country’s ambitions to deliver high tech medical diagnostics. DynamX is designed to improve the treatment of one of the most prevalent cardiovascular conditions, coronary artery disease.
The DynamX Bioadaptor is a metal implant with a drug-eluting bioresorbable polymer that supports the coronary artery during healing, with radial strength similar to drug-eluting stents (DES). Over six months, the polymer coating dissolves, uncaging the bioadaptor and freeing the artery to move with the natural expansion and contraction of the artery, unlike DES. This has been shown to (a) maintain the ability for positive adaptive remodeling, (b) restore vessel function, and (c) allow for the vessel’s return towards baseline angulation.
“Coronary arteries naturally have the ability to expand with disease progression in order to maintain blood flow to the heart,” said Antonio Colombo, MD, co-principal investigator of the DynamX Mechanistic Clinical Study and director, Cardiac Catheterization Laboratory, Columbus Hospital, Milan and Coordinators Cardiac Catheterization Laboratories GVM Group, Care and Research, Lugo (RA), Italy. “Drug-eluting stents cage the coronary arteries and hinder this physiological response. DynamX is the first metallic coronary artery implant to demonstrate positive adaptive remodeling of the vessel, enabling it to expand to accommodate disease progression. This may improve longer-term clinical outcomes.”
The bioadaptor is designed to address the 2% – 3% major adverse cardiac event rate that occur with drug-eluting stents each year without plateau.[3],[4],[5] The rigid design of a DES constrains, or “cages,” natural artery movement disabling its natural ability to accommodate disease progression, which has been associated with major adverse cardiac events (MACE).[6] Long-term studies have shown adverse event rates of 20% at 5 years3,4,5 and 40%-50% at 10 years.[7] Clinical studies have demonstrated that a DES prevents positive adaptive remodeling,[8] inhibits vessel compliance and dilation in response to the body’s changing blood flow needs,[9],[10] increases stresses on the vessel [11], and causes vessel straightening which has been associated with increased MACE.[12]
Studies have shown that the DynamX Bioadaptor improves vessel function in several ways. It enables the vessel to accommodate disease progression by increasing vessel area, maintaining lumen diameter and preserving the lumen area and therefore blood flow to the heart, known as positive adaptive remodeling.[13] DynamX restores vessel function and allows for normal vessel pulsatility and motion enabling it to provide more blood flow response to the body’s needs during physical activity. Its design also reduces stresses on the vessel and allows it to return to baseline angulation, which may reduce adverse events.
“We are launching DynamX in select countries in the Middle East, Asia, and other geographies at major centers of excellence, and Elixir is commencing multiple randomized clinical trials globally to build rigorous, clinically-based evidence for DynamX,” said Elixir Medical CEO Motasim Sirhan.
The first patient in the Middle East has been successfully treated with the DynamX Coronary Bioadaptor System by Arif Al Nooryani, Chief Executive Officer & Head of Cardiac Centre at Al Qassimi Hospital, Sharjah, United Arab Emirates.
MEDIA CONTACT: Lisa George/Ranya Al Bayoumi. Iris PR, Dubai, UAE. Tel: +9714 434 1207. Email: lisa@irispr.net / ranya@irispr.net
About Elixir Medical
Elixir Medical Corporation, a privately-funded company based in Milpitas, California, develops next-generation platforms to treat coronary artery disease that are designed to restore the adaptive remodeling and pulsatile motion capabilities of the blood vessel. The company’s mission is to transform the care of patients with heart and vascular disease through innovation.
The DynamX Sirolimus Eluting Coronary Bioadaptor System is CE Mark approved. Not available for sale in the USA.
[1] A Shehab, A S Bhagavathula, P5317
Prevalence of cardiovascular diseases in the Middle-East: systemic review and meta-analysis, European Heart Journal, Volume 40, Issue Supplement_1, October 2019, ehz746.0287, https://doi.org/10.1093/eurheartj/ehz746.0287. CVDs are responsible for 34% of all deaths in the middle east population
[2] “Coronary Heart Disease in Kuwait.” World Life Expectancy, https://www.worldlifeexpectancy.com/kuwait-coronary-heart-disease
[3] Iqbal J, Serruys PW, Silber S, et al. Comparison of zotarolimus- and everolimus-eluting coronary stents: final 5-year report of the RESOLUTE all-comers trial. Circ Cardiovasc Interv. 2015;8(6):e002230. doi:10.1161/CIRCINTERVENTIONS.114.002230
[4] Gada, H, Kirtane, A, Newman, W, et al. (2013). 5-Year Results of a Randomized Comparison of XIENCE V Everolimus-Eluting and TAXUS Paclitaxel-Eluting Stents. JACC. Cardiovascular interventions. 6. 10.1016/j.jcin.2013.07.009.
[5] Smits, PC, Valchojannis, GJ, McFadden, EP, et al. Final 5-Year Follow-Up of a Randomized Controlled Trial of Everolimus- and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice. J Am Coll Cardiol Intv. 2015 Aug, 8 (9) 1157-1165.
[6] Stone GW, Kimura T, Gao R, et al. Time-Varying Outcomes With the Absorb Bioresorbable Vascular Scaffold During 5-Year Follow-up: A Systematic Meta-analysis and Individual Patient Data Pooled Study. JAMA Cardiol. 2019;4(12):1261–1269. doi:10.1001/jamacardio.2019.4101
[7] Kufner S, Joner M, Thannheimer A, et al. Ten-year clinical outcomes from a trial of three limus-eluting stents with different polymer coatings in patients with coronary artery disease: results from the ISAR-TEST 4 randomized trial. Circulation 2019; 139:325–333. doi: 10.1161/CIRCULATIONAHA.118.038065.
[8] Nakamura M, Yock PG, Bonneau HN, et al. Impact of peri-stent remodeling on restenosis: a volumetric intravascular ultrasound study. Circulation. 2001;103(17):2130-2132. doi:10.1161/01.cir.103.17.2130
[9] Hamilos, M, Sarma, J, Ostojic, M, et al. (2008). Interference of Drug-Eluting Stents With Endothelium-Dependent Coronary Vasomotion Evidence for Device-Specific Responses. Circulation. Cardiovascular interventions. 1. 193-200. 10.1161/CIRCINTERVENTIONS.108.797928.
[10] Maier, W, Windercker, S, Kung, A, et al. Exercise-Induced Coronary Artery Vasodilation Is Not Impaired by Stent Placement. Circulation. 2002; 105(20): 2373-2377. doi: 10.1161/01.cir.0000016360.97819.44
[11] McDaniel MC, Samady H. The sheer stress of straightening the curves: biomechanics of bioabsorbable stents. JACC Cardiovasc Interv. 2011 Jul;4(7):800-2. doi: 10.1016/j.jcin.2011.05.008. PMID: 21777889.
[12] Gyöngyösi M, Yang P, Khorsand A, Glogar D. Longitudinal straightening effect of stents is an additional predictor for major adverse cardiac events. Austrian Wiktor Stent Study Group and European Paragon Stent Investigators. J Am Coll Cardiol. 2000;35(6):1580-1589. doi:10.1016/s0735-1097(00)00570-2
[13] Verheye S, Vrolix M, Montorfano M, et al. Twelve-month clinical and imaging outcomes of the upcaging DynamX Bioadaptor System. EuroIntervention 2020; Jaa-835, 2020, doi: 10.4244/EIJ-D-20-00763.
© Press Release 2020
Disclaimer: The contents of this press release was provided from an external third party provider. This website is not responsible for, and does not control, such external content. This content is provided on an “as is” and “as available” basis and has not been edited in any way. Neither this website nor our affiliates guarantee the accuracy of or endorse the views or opinions expressed in this press release.
The press release is provided for informational purposes only. The content does not provide tax, legal or investment advice or opinion regarding the suitability, value or profitability of any particular security, portfolio or investment strategy. Neither this website nor our affiliates shall be liable for any errors or inaccuracies in the content, or for any actions taken by you in reliance thereon. You expressly agree that your use of the information within this article is at your sole risk.
To the fullest extent permitted by applicable law, this website, its parent company, its subsidiaries, its affiliates and the respective shareholders, directors, officers, employees, agents, advertisers, content providers and licensors will not be liable (jointly or severally) to you for any direct, indirect, consequential, special, incidental, punitive or exemplary damages, including without limitation, lost profits, lost savings and lost revenues, whether in negligence, tort, contract or any other theory of liability, even if the parties have been advised of the possibility or could have foreseen any such damages.