PHOTO
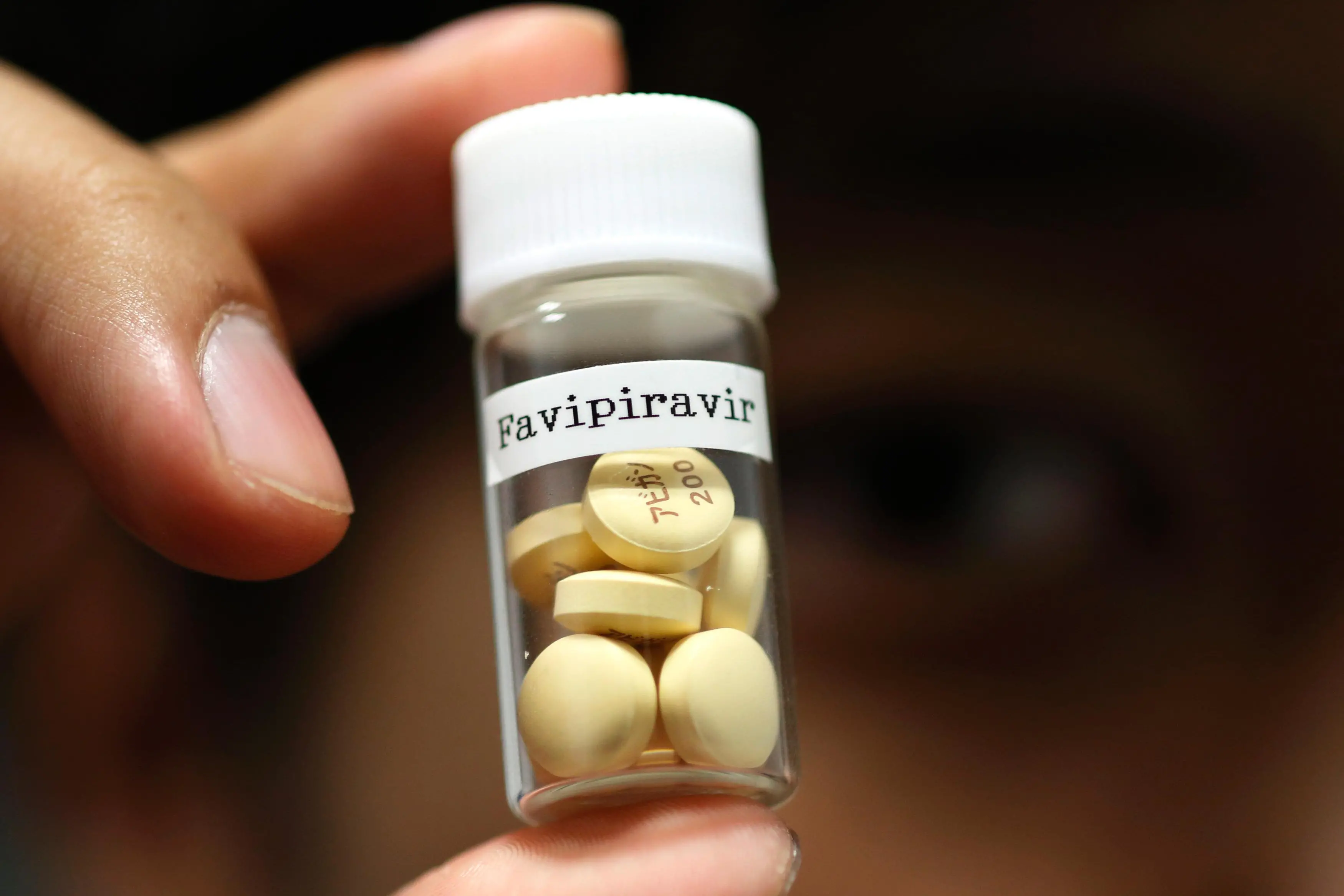
BENGALURU - Glenmark Pharmaceuticals Ltd said on Monday it would lower the price of its generic version of favipiravir, FabiFlu, to 75 rupees ($0.9983) per tablet for restricted emergency use in patients with mild-to-moderate COVID-19 symptoms in India.
Glenmark last month received Indian regulatory approval to make and sell anti-flu drug favipiravir, which is manufactured under the brand name Avigan by a unit of Japan's Fujifilm Holdings Corp.
Glenmark had priced FabiFlu at 103 rupees per tablet earlier, making it one of the cheapest COVID-19 treatment available in the country.
"Glenmark's price reduction aims to make FabiFlu further accessible for COVID-19 patients across the country," the company said in a statement.
The price reduction comes at a time when coronavirus cases are continuing to surge across India. The country registered a record increase in infections on Sunday, and has over 870,000 cases as of Monday, with the death toll at 23,174, according to federal health ministry data.
Social media users have also complained of a shortage of COVID-19 treatment in the nation.
A treatment course with FabiFlu would require a patient to take 122 tablets over 14 days, and will now cost 8,475 rupees ($112.80) per patient at the new price.
Meanwhile, Japanese researchers on Friday said a clinical trial of Fujifilm's Avigan yielded inconclusive results as a treatment for COVID-19.
Dr. Reddy's Laboratories Ltd has entered into a deal with Fujifilm to sell Avigan globally excluding Japan, China and Russia.
Mumbai-based Glenmark has also commenced a post marketing surveillance study with FabiFlu to monitor its efficacy and safety in 1,000 patients that are prescribed with the oral antiviral, the company said.
($1 = 75.1300 Indian rupees)
(Reporting by Anuron Kumar Mitra in Bengaluru; Editing by Rashmi Aich) ((AnuronKumar.Mitra@thomsonreuters.com; +91 99863 58469;))