PHOTO
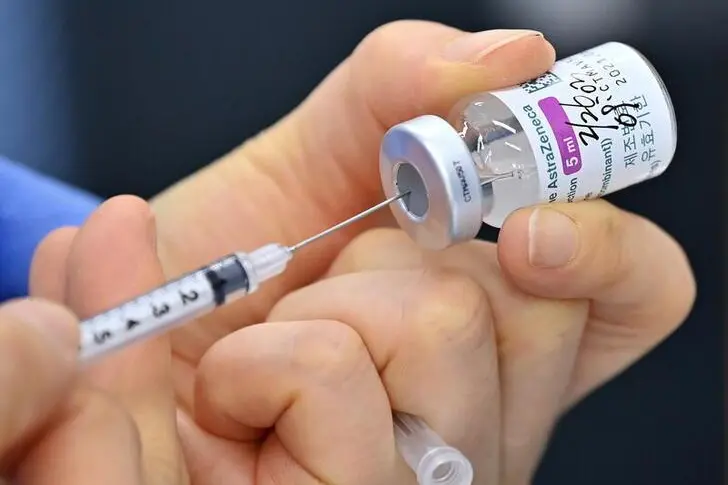
SEOUL - South Korea said on Wednesday it will temporarily suspend providing AstraZeneca's COVID-19 vaccine to people below 60 amid a European review, while approving a Johnson & Johnson shot in a bid to speed up its inoculation rollout.
The European Medical Agency (EMA) is due to announce the results of a review on whether some cases of blood clotting in adults may be linked to the AstraZeneca shot.
The announcement is due at 1400 GMT.
Global controversy over the efficacy and side-effects of some COVID-19 vaccines has caused some delays in South Korea's vaccination campaign, which kicked off in late February with the goal of reaching herd immunity in November.
AstraZeneca has said previously its studies have found no higher risk of clots because of its vaccine, millions of doses of which have been administered worldwide. The World Health Organization and EMA have both said the benefits outweigh the risks.
South Korea's food and drug safety ministry said on Wednesday it had granted final approval for Johnson & Johnson's (J&J) vaccine after a panel of experts ruled the single-dose shot was safe and effective.
J&J is the third COVID-19 vaccine maker to be authorised in South Korea, after AstraZeneca and Pfizer /BioNTech , both of which require two doses.
Authorities are seeking to speed up a national vaccination campaign despite global supply shortages while expanding preemptive testing and tracing efforts amid concerns over a potential fourth wave of infections.
The Korea Disease Control and Prevention Agency (KDCA) reported 668 new cases for Tuesday, the highest level since Jan. 8, with clusters developing in kindergartens, saunas, bars and churches, mostly in the greater Seoul area.
To date, total infections stand at 106,898, with 1,756 deaths.
"If the fourth wave of infections becomes a reality, a disruption to vaccination would be inevitable, as well as dealing a big blow to our economy," Prime Minister Chung Sye-kyun told a government meeting.
Health officials called on the public to refrain from non-essential gatherings and strictly log visits to crowded venues to help with epidemiological work in case of any outbreak.
They also said they would announce new social distancing rules on Friday after discussion with experts and local governments.
The country has so far administered over a million doses of vaccine among medical workers and high-risk groups since the start of its inoculation drive in February, yet authorities face a backlash for relying on the global vaccine-sharing programme COVAX, which has run into delays.
(Additional reporting and writing by Hyonhee Shin; Editing by Kenneth Maxwell and Nick Macfie) ((Sangmi.Cha@thomsonreuters.com; +82 2 3704 5646; Reuters Messaging: sangmi.cha.thomsonreuters.com@reuters.net))